Chemical element Niobium
General
Information
niobium
chemical element
Niobium (pronounced |nī'ōbēəm|) (Greek
mythology: Niobe, daughter of Tantalus), or columbium (|kə'ləmbēəm|), is the chemical
element with the symbol Nb and the atomic number 4. A rare, soft, grey, ductile
transition metal, niobium is found in the minerals pyrochlore, the main
commercial source for niobium, and columbite.has physical and chemical
properties similar to those of the element tantalum, and the two are therefore
difficult to distinguish. The English chemist Charles Hatchett reported a new
element similar to tantalum in 1801, and named it columbium. In 1809, the
English chemist William Hyde Wollaston wrongly concluded that tantalum and
columbium were identical. The German chemist Heinrich Rose determined in 1846
that tantalum ores contain a second element, which he named niobium. In 1864
and 1865, a series of scientific findings clarified that niobium and columbium
were the same element (as distinguished from tantalum), and for a century both
names were used interchangeably. The name of the element was officially adopted
as niobium in 1949.was not until the early 20th century that niobium was first
used commercially. Brazil is the leading producer of niobium and ferroniobium,
an alloy of niobium and iron. Niobium is used mostly in alloys, the largest
part in special steel such as that used in gas pipelines. Although alloys
contain only a maximum of 0.1%, that small percentage of Niobium improves the
strength of the steel. The temperature stability of niobium-containing
superalloys is important for its use in jet engines and rocket engines. Niobium
is used in various superconducting materials. These superconducting alloys,
also containing titanium and tin, are widely used in the superconducting
magnets of MRI scanners. Other applications of niobium include its use in
welding, nuclear industries, electronics, optics, numismatics and jewellery. In
the last two applications, niobium's low toxicity and ability to be colored by
anodisation are particular advantages.
History
was discovered by the English chemist Charles Hatchett in
1801. He found a new element in a mineral sample that had been sent to England
from Massachusetts in 1734 by a John Winthrop, and named the mineral columbite
and the new element columbium after Columbia, the poetical name for America.
The columbium discovered by Hatchett was probably a mixture of the new element
with tantalum., there was considerable confusion over the difference between
columbium (niobium) and the closely related tantalum. In 1809, the English
chemist William Hyde Wollaston compared the oxides derived from both
columbium-columbite, with a density 5.918 g/cm³, and tantalum-tantalite,
with a density 7.935 g/cm³, and concluded that the
two oxides, despite the significant difference in density, were identical; thus
he kept the name tantalum. This conclusion was disputed in 1846 by the German
chemist Heinrich Rose, who argued that there were two different elements in the
tantalite sample, and named them after children of Tantalus: niobium (from
Niobe, the goddess of tears), and pelopium (from Pelops). This confusion arose
from the minimal observed differences between tantalum and niobium. Both
tantalum and niobium react with chlorine and traces of oxygen, including
atmospheric concentrations, with niobium forming two compounds: the white
volatile niobium pentachloride (NbCl5) and the non-volatile niobium oxychloride
(NbOCl3). The claimed new elements pelopium, ilmenium and dianium were in fact
identical to niobium or mixtures of niobium and tantalum.differences between
tantalum and niobium were unequivocally demonstrated in 1864 by Christian
Wilhelm Blomstrand, and Henri Etienne Sainte-Claire Deville, as well as Louis
J. Troost, who determined the formulas of some of the compounds in 1865 and
finally by the Swiss chemist Jean Charles Galissard de Marignac in 1866, who
all proved that there were only two elements. These discoveries did not stop
scientists from publishing articles about ilmenium until 1871. De Marignac was
the first to prepare the metal in 1864, when he reduced niobium chloride by
heating it in an atmosphere of hydrogen.de Marignac was able to produce
tantalum-free niobium on an increased scale by 1866, it was not until the early
20th century that niobium was first used commercially, in incandescent lamp
filaments. This use quickly became obsolete through the replacement of niobium
with tungsten, which has a higher melting point and thus is preferable for use
in incandescent lamps. The discovery that niobium improves the strength of
steel was made in the 1920s, and this remains its predominant use. In 1961 the
American physicist Eugene Kunzler and coworkers at Bell Labs discovered that
niobium-tin continues to exhibit superconductivity in the presence of strong
electric currents and magnetic fields, making it the first material known to
support the high currents and fields necessary for making useful high-power
magnets and electrically powered machinery. This discovery would allow-two
decades later-the production of long multi-strand cables that could be wound
into coils to create large, powerful electromagnets for rotating machinery,
particle accelerators, or particle detectors.(symbol Cb) was the name
originally given to this element by Hatchett, and this name remained in use in
American journals-the last paper published by American Chemical Society with
columbium in its title dates from 1953-while niobium was used in Europe. To end
this confusion, the name niobium was chosen for element 41 at the 15th
Conference of the Union of Chemistry in Amsterdam in 1949. A year later this
name was officially adopted by the International Union of Pure and Applied
Chemistry (IUPAC) after 100 years of controversy, despite the chronological
precedence of the name Columbium. The latter name is still sometimes used in US
industry. This was a compromise of sorts; the IUPAC accepted tungsten instead
of wolfram, in deference to North American usage; and niobium instead of
columbium, in deference to European usage. Not everyone agreed, and while many
leading chemical societies and government organizations refer to it by the
official IUPAC name, many leading metallurgists, metal societies, and the
United States Geological Survey still refer to the metal by the original "columbium".
is a lustrous, grey, ductile, paramagnetic metal in group 5
of the periodic table (see table to right), although it has an atypical
configuration in its outermost electron shells compared to the rest of the
members. (This can be observed in the neighborhood of niobium (41), ruthenium
(44), rhodium (45), and palladium (46).)
metal takes on a bluish tinge when exposed to air at room
temperature for extended periods. Despite presenting a high melting point in
elemental form (2,468 °C), it has a low density in comparison to other
refractory metals. Furthermore, it is corrosion resistant, exhibits
superconductivity properties, and forms dielectric oxide layers. These
properties- especially the superconductivity -are strongly dependent on the purity
of the niobium metal. When very pure, it is comparatively soft and ductile, but
impurities make it harder.is slightly less electropositive and smaller than its
predecessor in the periodic table, zirconium, while it is virtually identical
in size to the heavier tantalum as a consequence of the lanthanide contraction.
As a result, niobium's chemical properties are very similar to the chemical
properties of tantalum, which appears directly below niobium in the periodic
table. Although its corrosion resistance is not as outstanding as that of
tantalum, its lower price and greater availability make niobium attractive for
less exact uses such as linings in chemical plants.
of
niobium
occurring niobium (Nb) is composed of one stable isotope
(Nb-93). The most stable radioisotopes are Nb-92 with a half-life of 34.7
million years, Nb-94 (half life: 20300 years), and Nb-91 with a half life of
680 years. There is also a meta state at 31 keV whose half-life is 16.13 years.
Twenty three other radioisotopes have been characterized. Most of these have
half lives that are less than two hours except Nb-95 (35 days), Nb-96 (23.4
hours) and Nb-90 (14.6 hours). The primary decay mode before the stable Nb-93
is electron capture and the primary mode after is beta emission with some
neutron emission occurring in the first mode of the two mode decay of Nb-104,
109 and 110.Nb-95 (35 days) and Nb-97 (72 minutes) and heavier isotopes
(halflives in seconds) are fission products in significant quantity, as the
other isotopes are shadowed by stable or very long-lived (Zr-93) isotopes of
the preceding element zirconium from production via beta decay of neutron-rich
fission fragments. Nb-95 is the decay product of Zr-95 (64 days), so
disappearance of Nb-95 in used nuclear fuel is slower than would be expected
from its own 35 day halflife alone. Tiny amounts of the other isotopes may be
produced as direct fission products.atomic mass: 92.90638(2) u.
is in many ways similar to its predecessors in group 5. It
reacts with most nonmetals at high temperatures: niobium reacts with fluorine
at room temperature, with chlorine and hydrogen at 200 °C, and with nitrogen at
400 °C, giving products that are frequently interstitial and nonstoichiometric.
The metal begins to oxidize in air at 200 °C, and is resistant to corrosion by
fused alkalis and by acids, including aqua regia, chlorhydric, sulfuric, nitric
and phosphoric acids. Niobium is attacked by hot, concentrated mineral acids,
such as fluorhydric acid and fluorhydric/nitric acid mixtures. Although niobium
exhibits all the formal oxidation states from +5 down to -1, its most stable
state is +5.
Niobium is able to form oxides with the oxidation
states +5 (Nb2O5), +4 (NbO2) and +3 (Nb2O3), as well as with the rarer
oxidation state +2 (NbO). The most stable oxidation state is +5, the pentoxide
which, along with the dark green non-stoichiometric dioxide, is the most common
of the oxides. Niobium pentoxide is used mainly in the production of
capacitors, optical glass, and as starting material for several niobium
compounds. The compounds are created by dissolving the pentoxide in basic
hydroxide solutions or by melting it in another metal oxide. Examples are
lithium niobate (LiNbO3) and lanthanum niobate (LnNbO4). In the lithium
niobate, the niobate ion NbO3− is not alone but part of a perovskite-like
structure, while the lanthanum niobate contains lone 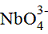
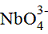 ions.
Lithium niobate, which is a ferroelectric, is used extensively in mobile telephones
and optical modulators, and for the manufacture of surface acoustic wave
devices. It belongs to the ABO3 structure ferroelectrics like lithium tantalate
and barium titanate.forms halogen compounds in the oxidation states of +5, +4,
and +3 of the type NbX5, NbX4, and NbX3, although multi-core complexes and
substoichiometric compounds are also formed. Niobium pentafluoride (NbF5) is a
white solid with a melting point of 79.0 °C and niobium pentachloride (NbCl5)
is a yellowish-white solid (see image at left) with a melting point of 203.4
°C. Both are hydrolyzed by water and react with additional niobium at elevated
temperatures by forming the black and highly hygroscopic niobium tetrafluoride
(NbF4) and niobium tetrachloride (NbCl4). While the trihalogen compounds can be
obtained by reduction of the pentahalogens with hydrogen, the dihalogen
compounds do not exist. Spectroscopically, the monochloride (NbCl) has been
observed at high temperatures. The fluorides of niobium can be used after its
separation from tantalum. The niobium pentachloride is used in organic
chemistry as a Lewis acid in activating alkenes for the carbonyl-ene reaction
and the Diels-Alder reaction. The pentachloride is also used to generate the
organometallic compound niobocene dichloride ((C5H5)2NbCl2), which in turn is
used as a starting material for other organoniobium compounds.binary compounds
of niobium include niobium nitride (NbN), which becomes a superconductor at low
temperatures and is used in detectors for infrared light, and niobium carbide,
an extremely hard, refractory, ceramic material, commercially used in tool bits
for cutting tools. The compounds niobium-germanium (Nb3Ge) and niobium-tin
(Nb3Sn), as well as the niobium-titanium alloy, are used as a type II
superconductor wire for superconducting magnets. Niobium sulfide as well as a
few interstitial compounds of niobium with silicon are also known.
ions.
Lithium niobate, which is a ferroelectric, is used extensively in mobile telephones
and optical modulators, and for the manufacture of surface acoustic wave
devices. It belongs to the ABO3 structure ferroelectrics like lithium tantalate
and barium titanate.forms halogen compounds in the oxidation states of +5, +4,
and +3 of the type NbX5, NbX4, and NbX3, although multi-core complexes and
substoichiometric compounds are also formed. Niobium pentafluoride (NbF5) is a
white solid with a melting point of 79.0 °C and niobium pentachloride (NbCl5)
is a yellowish-white solid (see image at left) with a melting point of 203.4
°C. Both are hydrolyzed by water and react with additional niobium at elevated
temperatures by forming the black and highly hygroscopic niobium tetrafluoride
(NbF4) and niobium tetrachloride (NbCl4). While the trihalogen compounds can be
obtained by reduction of the pentahalogens with hydrogen, the dihalogen
compounds do not exist. Spectroscopically, the monochloride (NbCl) has been
observed at high temperatures. The fluorides of niobium can be used after its
separation from tantalum. The niobium pentachloride is used in organic
chemistry as a Lewis acid in activating alkenes for the carbonyl-ene reaction
and the Diels-Alder reaction. The pentachloride is also used to generate the
organometallic compound niobocene dichloride ((C5H5)2NbCl2), which in turn is
used as a starting material for other organoniobium compounds.binary compounds
of niobium include niobium nitride (NbN), which becomes a superconductor at low
temperatures and is used in detectors for infrared light, and niobium carbide,
an extremely hard, refractory, ceramic material, commercially used in tool bits
for cutting tools. The compounds niobium-germanium (Nb3Ge) and niobium-tin
(Nb3Sn), as well as the niobium-titanium alloy, are used as a type II
superconductor wire for superconducting magnets. Niobium sulfide as well as a
few interstitial compounds of niobium with silicon are also known.
to estimates, niobium is 33rd on the list of the most common
elements in the Earth’s crust with 20 ppm. The abundance on Earth should be
much greater, but the “missing” niobium may be located in the Earth’s core due
to the metal's high density. The free element is not found in nature, but it
does occur in minerals. Minerals that contain niobium often also contain
tantalum, for example, columbite ((Fe,Mn)(Nb,Ta)2O6), columbite-tantalite (or
coltan, (Fe,Mn)(Ta,Nb)2O6) and pyrochlore ((Na,Ca)2Nb2O6(OH,F)).
Columbite-tantalite minerals are most usually found as accessory minerals in
pegmatite intrusions, and in alkaline intrusive rocks. Less common are the
niobates of calcium, uranium, thorium and the rare earth elements such as
pyrochlore and euxenite ((Y,Ca,Ce,U,Th)(Nb,Ta,Ti)2O6). These large deposits of
niobium have been found associated with carbonatites (carbonate-silicate igneous
rocks) and as a constituent of pyrochlore.two largest deposits of pyrochlore
were found in the 1950s in Brazil and Canada, and both countries are still the
major producers of niobium mineral concentrates. The largest deposit is hosted
within a carbonatite intrusion at Araxá, Minas Gerais Brazil,
owned by CBMM (Companhia Brasileira de Metalurgia e Mineração); the other deposit is
located at Catalão, Goiás owned by Anglo American
plc (through its subsidiary Mineração Catalão), also hosted within a
carbonatite intrusion. Altogether these two Brazilian mines produce around 75%
of world supply. The third largest producer of niobium is the
carbonatite-hosted Niobec Mine, Saint-Honoré near Chicoutimi, Quebec
owned by Iamgold Corporation Ltd, which produces around 7% of world
supply.though unexploited resources are located in Nigeria, Democratic Republic
of Congo, Malawi, Australia and in Russia.
the separation from the other minerals, the mixed oxides of
tantalum Ta2O5 and niobium Nb2O5 are obtained. The first step in the processing
is the reaction of the oxides with hydrofluoric acid:
O5 + 14HF → 2H2[TaF7] + 5H2O, andO5 + 10HF →
2H2[NbOF5] + 3H2O
first industrial scale separation, developed by de Marignac,
used the difference in solubility between the complex niobium and tantalum
fluorides, dipotassium oxypentafluoroniobate monohydrate (K2[NbOF5]·H2O) and
dipotassium heptafluorotantalate (K2[TaF7]) in water. Newer processes use the
liquid extraction of the fluorides from aqueous solution by organic solvents
like cyclohexanone. The complex niobium and tantalum fluorides are extracted
separately from the organic solvent with water and either precipitated by the
addition of potassium fluoride to produce a potassium fluoride complex, or precipitated
with ammonia as the pentoxide:
[NbOF5] + 2KF → K2[NbOF5]↓ + 2HF, then
H2[NbOF5] + 10NH4OH → Nb2O5↓ + 10NH4F + 7H2O
methods are used for the reduction to metallic niobium. The
electrolysis of a molten mixture of K2[NbOF5] and sodium chloride is one; the
other is the reduction of the fluoride with sodium. With this method niobium
with a relatively high purity can be obtained. In large scale production the
reduction of Nb2O5 with hydrogen or carbon is used. In the process involving
the aluminothermic reaction a mixture of iron oxide and niobium oxide is
reacted with aluminum:
Nb2O5 + Fe2O3 + 12Al → 6Nb + 2Fe + 6Al2O3
enhance the reaction, small amounts of oxidizers like sodium
nitrate are added. The result is aluminum oxide and ferroniobium, an alloy of
iron and niobium used in the steel production. The ferroniobium contains
between 60 and 70% of niobium. Without addition of iron oxide, aluminothermic
process is used for the production of niobium. Further purification is
necessary to reach the grade for superconductive alloys. Electron beam melting
under vacuum is the method used by the two major distributors of niobium.United
States Geological Survey estimates that the production increased from 38,700
metric tonnes in 2005 to 44,500 tonnes in 2006. The world wide resources are
estimated to be 4,400,000 tonnes. During the ten year period between 1995 and
2005, the production more than doubled starting from 17,800 tonnes in 1995.
Applications
is estimated that out of 44,500 metric tons of niobium mined in
2006, 90% ended up in the production of high-grade structural steel, followed
by its use in superalloys. The use of niobium alloys for superconductors and in
electronic components account only for a small share of the production.is an
effective microalloying element for steel. Adding niobium to the steel causes
the formation of niobium carbide and niobium nitride within the structure of
the steel. These compounds improve the grain refining, retardation of
recrystallization, and precipitation hardening of the steel. These effects in
turn increase the toughness, strength, formability, and weldability of the
microalloyed steel. Microalloyed stainless steels have a niobium content of
less than 0.1%. It is an important alloy addition to high strength low alloy
steels which are widely used as structural components in modern automobiles.
These niobium containing alloys are strong and are often used in pipeline
construction.amounts of the element, either in its pure form or in the form of
high-purity ferroniobium and nickel niobium, are used in nickel-, cobalt-, and
iron-base superalloys for such applications as jet engine components, gas
turbines, rocket subassemblies, and heat resisting and combustion equipment.
Niobium precipitates a hardening γ''-phase within the grain
structure of the superalloy. The alloys contain up to 6.5% niobium. One example
of a nickel-based niobium-containing superalloy is Inconel 718, which consists
of roughly 50% nickel, 18.6% chromium, 18.5% iron, 5% niobium, 3.1% molybdenum,
0.9% titanium, and 0.4% aluminum. These superalloys are used, for example, in
advanced air frame systems such as those used in the Gemini program.alloy used
for liquid rocket thruster nozzles, such as in the main engine of the Apollo
Lunar Modules, is C103, which consists of 89% niobium, 10% hafnium and 1%
titanium. Another niobium alloy was used for the nozzle of the Apollo Service
Module. As niobium is oxidized at temperatures above 400 °C, a protective
coating is necessary for these applications to prevent the alloy from becoming
brittle.becomes a superconductor when lowered to cryogenic temperatures. At
atmospheric pressure, it has the highest critical temperature of the elemental
superconductors: 9.2 K. Niobium has the largest magnetic penetration depth of
any element. In addition, it is one of the three elemental Type II
superconductors, along with vanadium and technetium. Niobium-tin and
niobium-titanium alloys are used as wires for superconducting magnets capable
of producing exceedingly strong magnetic fields. These superconducting magnets
are used in magnetic resonance imaging and nuclear magnetic resonance
instruments as well as in particle accelerators. For example, the Large Hadron
Collider uses 600 metric tons of superconducting strands, while the International
Thermonuclear Experimental Reactor is estimated to use 600 metric tonnes of
Nb3Sn strands and 250 metric tonnes of NbTi strands. In 1992 alone,
niobium-titanium wires were used to construct more than 1 billion US dollars
worth of clinical magnetic resonance imaging systems.is used as a precious
metal in commemorative coins, often with silver or gold. For example, Austria
produced a series of silver niobium euro coins starting in 2003; the color in
these coins is created by diffraction of light by a thin oxide layer produced
by anodizing. In 2008, six coins are available showing a broad variety of
colors in the centre of the coin: blue, green, brown, purple, violet, or
yellow. Two more examples are the 2004 Austrian 25 euro 150 Years Semmering
Alpine Railway commemorative coin, and the 2006 Austrian 25 euro European
Satellite Navigation commemorative coin. Latvia produced a similar series of
coins starting in 2004, with one following in 2007.and some niobium alloys are
used in medical devices such as pacemakers, because they are physiologically
inert (and thus hypoallergenic). Niobium treated with sodium hydroxide forms a
porous layer that aids osseointegration. Along with titanium, tantalum, and
aluminum, niobium can also be electrically heated and anodized, resulting in a
wide array of colors using a process known as reactive metal anodizing which is
useful in making jewelry. The fact that niobium is hypoallergenic also benefits
its use in jewelry.arc-tube seals of high pressure sodium vapor lamps are made
from niobium, or niobium with 1% of zirconium, because niobium has a very
similar coefficient of thermal expansion to the sintered alumina arc tube
ceramic, a translucent material which resists chemical attack or reduction by
the hot liquid sodium and sodium vapor contained inside the operating lamp. The
metal is also used in arc welding rods for some stabilized grades of stainless
steel.was evaluated as a cheaper alternative to tantalum in capacitors, but
tantalum capacitors are still predominant. Niobium is added to glass in order
to attain a higher refractive index, a property of use to the optical industry
in making thinner corrective glasses. The metal has a low capture cross-section
for thermal neutrons; thus it is used in the nuclear industries.Superconducting
Radio Frequency (RF) cavities used in the free electron lasers TESLA and XFEL
are made from pure niobium.high sensitivity of superconducting niobium nitride
bolometers make them an ideal detector for electromagnetic radiation in the THz
frequency band. These detectors were tested at the Heinrich Hertz Submillimeter
Telescope, the South Pole Telescope, the Receiver Lab Telescope, and at APEX
and are now used in the HIFI instrument on board the Herschel Space
Observatory.
has no known biological role. While niobium dust is an eye
and skin irritant and a potential fire hazard, elemental niobium on a larger
scale is physiologically inert (and thus hypoallergenic) and harmless. It is
frequently used in jewelry and has been tested for use in some medical
implants.containing compounds are rarely encountered by most people, but some
are toxic and should be treated with care. The short and long term exposure to
niobates and niobium chloride, two chemicals that are water soluble, have been
tested in rats. Rats treated with a single injection of niobium pentachloride
or niobates show a median lethal dose (LD50) between 10 and 100 mg/kg. For oral
administration the toxicity is lower; a study with rats yielded a LD50 after
seven days of 940 mg/kg.
Загальна
інформація
Ніобій (грецька міфологія: Ніоба, дочка Тантала), або
Колумбій, - хімічний елемент з символом Nb і атомним номером 4. Рідкісний,
м’який, сірий, тягучий транзитивний метал, Ніобій знаходиться у мінералах
пірохлору, головного промислового джерела Ніобію, і в колумбітах.
Ніобій промислово не використовувався до початку XX ст. Бразилія - провідний виробник
ніобію і фероніобію, сплаву ніобію і заліза. Ніобій використовується в
основному у сплавах, найбільше у спеціальних сталях, як у тих, що призначені
для газових труб. Хоча сплави вміщують його лише 0,1% і менше, така мала частка
ніобію покращує міцність сталі. Температурна стійкість ніобієвмісних
суперсплавів є важливою для використання їх у літакових та ракетних двигунах.
Ніобій використовується у різних надпровідних матеріалах. Ці надпровідні
сплави, що вміщують також титан і олово, широко використовуються у надпровідних
магнітах магніторезонансних (MRI) сканерів. Інші застосування ніобію включають його використання у
зварюванні, ядерній промисловості, електроніці, оптиці, монетах і ювелірних
виробах. У двох останніх використаннях окремими перевагами ніобію є його низька
токсичність і здатність змінювати колір при анодному осадженні.
ductile - тягучий, в’язкий, пластичний.
transition - процес або період зміни стану; перехід між
квантовими станами атома або частинки, з поглинанням або випромінюванням
радіації.
clarify - прояснювати, пояснювати.
tin - олово.
MRI scanner - магніторезонансний сканер.
weld - зварювати.
numismatics - нумізматика, вивчення монет, паперової валюти і
медалей; колекція монет, валюти, медалей.
chlorine - Хлор (елемент і газ).
volatile - леткий, що легко випаровується; нестійкий;
неврівноважений; оперативний (про комп’ютерну пам’ять).
unequivocally - безсумнівно; однозначно.
all prove - повністю довести.
reduce - відновлювати(ся) (про атом - приєднувати електрон іншого
атома); сполучати з Гідрогеном.
incandescent - що світиться від нагріву; дуже розлючений;
надзвичайної якості, чудовий.
filament - нитка (в т. ч. нитка розжарення у лампі).
tungsten - вольфрам.
exhibit - демонструвати.
coil - бухта; виток; котушка.
controversy - суперечка.
sort - характер.
lustrous - блискучий, сяючий.
paramagnetic - немагнітний.
tinge - відтінок.
refractory - впертий; незмінний; жаростійкий.
contraction - зменшення; скорочення.
line - (серед багатьох інших значень) покривати зсередини.
meta- - перехідний
decay - гнити; руйнуватися; занепадати; змінюватися внаслідок
випромінювання радіації.
fluorine - фтор.
chlorine - хлор.
interstitial - проміжний.
nonstoichiometric - нестехіометричний (в кількості, що не підходить для
ідеальних умов реакції).
fuse - змішувати; захищати (запобіжником); спрацювати (про
захист).
alkali - луг.
aqua regia - суміш нітратної (азотної) та хлорної (соляної) кислот,
"царська водка".
attack - тут: руйнувати.
perovskite - жовтий, коричневий або чорний мінерал, складається
переважно з титанату кальцію.
ferroelectric - полярний.
pegmatite - крупнокристалічний граніт або вулканічні камені з
сантиметровими або метровими кристалами.